Uvulopalatoplasty is a surgical technique used to reduce or eliminate snoring. For over three decades, laser-assisted uvulopalatoplasty (LAUP) has been used to treat snoring and obstructive sleep apnea.
It is an out-patient procedure, in which a laser is used to remove parts or all of the uvula at the rear of the mouth.
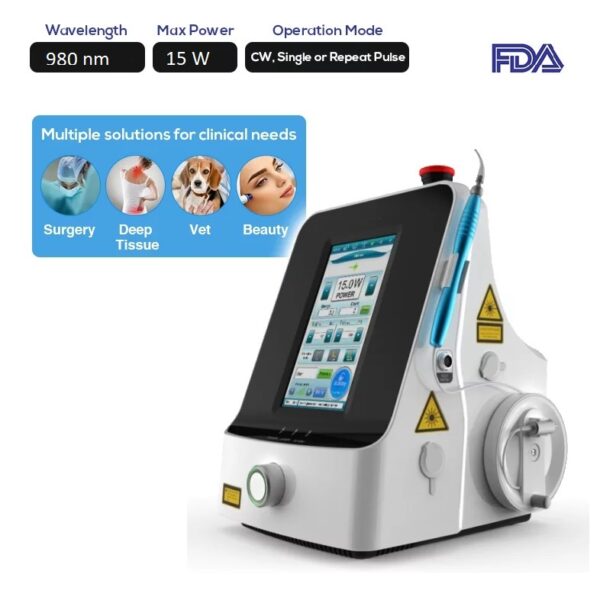
Non-surgical laser-guided uvulopalatoplasty surgeries frequently necessitate the use of a 980 nm laser wavelength to ensure a successful outcome.
The FDA Portable Surgery Diode Laser System LASER-1.2B is particularly developed to satisfy those criteria, operating at a wavelength of 980 nm with a maximum power of 15W.
These characteristics are precisely what non-surgical Uvulopalatoplasty treatment/surgery need.
To explain in more detail, the tissue components haemoglobin and melanin interact more with the blue laser light even at reduced power.
Accordingly, this allows LASER-1.2B to perform better and softer bloodless, pain-free uvula cuttings.
The LASER-1.2B is also highly recommended by doctors because it provides excellent alignment and precise sighting during surgery. Its green targeting laser is to thank for this.
Further, in order to guarantee better results, the device is supported with a fibre guide laser.
To begin with, it is suitable for a variety of endoscopic applications. Second, these particular fibres are sterilisable, which eliminates any possible cross-infection while ensuring a clean and bloodless operational region that lowers any potential heat damages.
Moreover, the complete absence of water absorption in the device helps drastically reduce the overheating of the surrounding tissues.
This should eliminate any possible after-effects which, in turn, will reinforce the laser treatment’s efficacy.
The LASER-1.2B ensures improved uvula cutting efficacy, far more than that attained with infrared lasers, thanks to all of these qualities and more.
The number of laser treatments in correlation to Uvulopalatoplasty has been constantly rising during the last years.
Thanks to the FDA Portable 980nm Surgery Laser System LASER-1.2B, it is now feasible to perform a large number of laser-guided bloodless Uvulopalatoplasty procedures.
Reference: Laser-assisted uvulopalatoplasty (LAUP) complications and side effects: a systematic review
Disclaimer: Although the information we provide is used by different doctors and medical staff to perform their procedures and clinical applications, the information contained in this article is for consideration only. SIFLASER is not responsible neither for the misuse of the device nor for the wrong or random generalizability of the device in all clinical applications or procedures mentioned in our articles. Users must have the proper training and skills to perform the procedure with each Laser System.
The products mentioned in this article are only for sale to medical staff (doctors, nurses, certified practitioners, etc.) or to private users assisted by or under the supervision of a medical professional.